Do All Olfactory Cortices Send Feedback Projections to the Olfactory Bulb?
Pablo S. Villar is currently a PhD Candidate in the Biological Sciences graduate program, University of Maryland, College Park, Maryland. Follow them on Twitter @pvillardr
Perception of sensory stimuli involves the reciprocal flow of neural signals from early processing centers to higher-order brain areas. Thus, top-down projections influence afferent sensory signals by acting on initial processing stages. Olfactory sensory neurons, which sense volatile odor molecules in the nose, send their axons to the olfactory bulb (OB), the first brain area where odor information is processed. The OB receives a large number of centrifugal projections from multiple brain areas. Centrifugal projections greatly outnumber afferent projections and include feedback projections from the olfactory cortex (OC) and neuromodulatory projections from various brain regions. The OC includes several areas that receive direct projections from mitral and tufted cells (M/TCs), which are the OB output neurons. Such OC areas include the anterior olfactory nucleus (AON), the piriform cortex (PC), and the olfactory tubercle (OT).
Studies have investigated how excitatory feedback projections from the OC modulate OB processing. For example, it has been proposed that PC feedback signals decorrelate the activity of M/TCs, allowing separation of odor representations. Similarly, research also suggests that AON feedback innervation modulates the timing of action potentials in M/TCs, which contributes to the reduction of background noise during odor responses. In contrast, the role of the OT in shaping early odor processing has received less attention. Similar to the PC and AON, the OT receives direct inputs from the OB output neurons. Previous studies indicated that similar to other regions of the OC, the OT sends direct feedback projections to the OB. In their eNeuro publication, in’t Zandt and colleagues performed a detailed analysis of the neuroanatomical connections of the OB with olfactory-related areas.
Three types of labeling experiments were performed in 6- to 12-week old mice (n = 19 male, n = 4 female). First, the authors microinjected fluorescent retrobead particles in the OB. These particles are endocytosed by axons and accumulate in the cell bodies of the OB-projecting neurons. As expected, PC and AON were readily labeled; however, no particles accumulated in OT neurons. Second, the absence of OT feedback projections to the OB was further confirmed by using retrograde adenoviruses to express green fluorescent protein. These viral particles efficiently infect neurons by their axon terminals, followed by retrograde transport to the soma where the fluorescent reporter is expressed. A third strategy to test for OT-OB direct connections included the use of a reporter mouse line (Ai9) that expresses the fluorophore tdTomato in the presence of the Cre recombinase. Cre recombinase expression was driven by a viral infection in the OB using a retrograde adenovirus. Again, this approach showed the labeling of multiple cortical and subcortical areas known to innervate the OB, yet tdTomato was not observed in OT neurons (Figure 1).
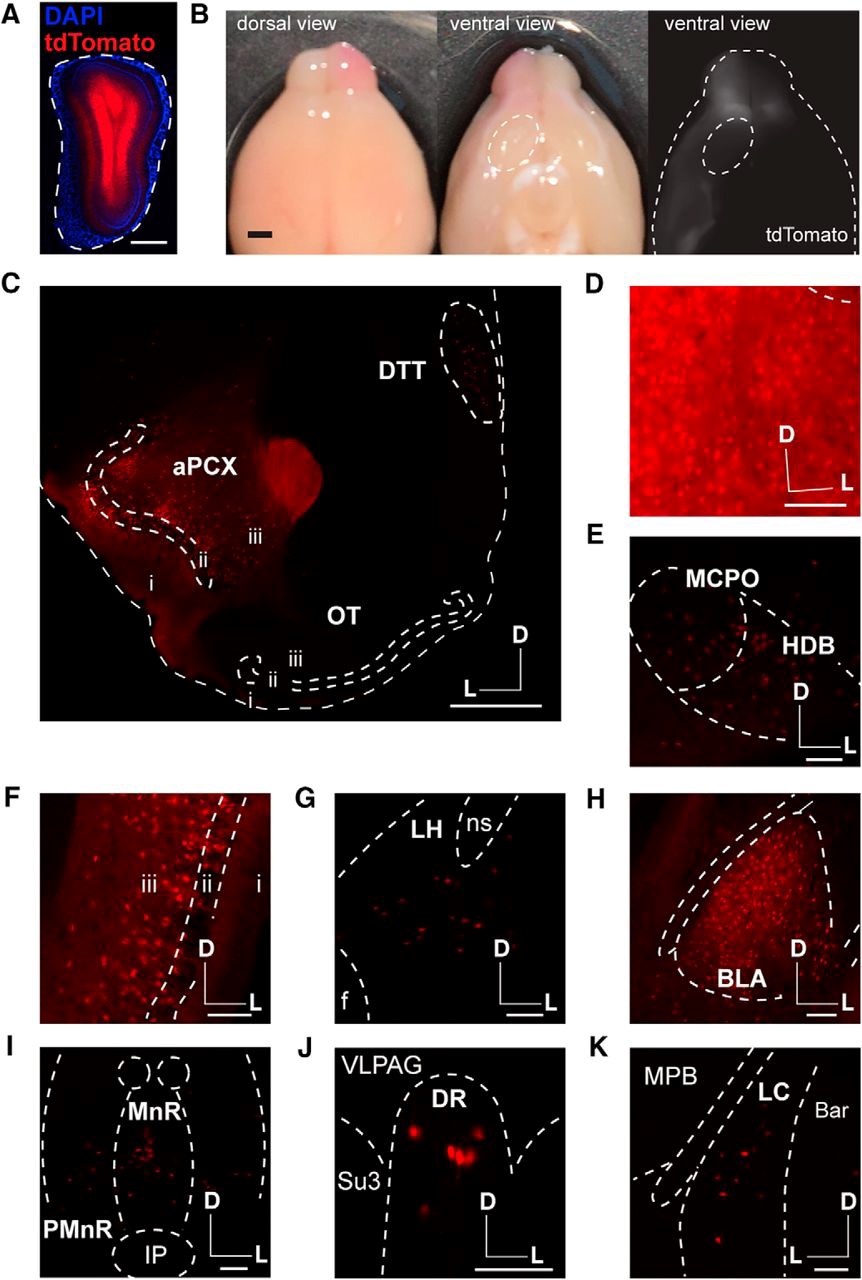
In their publication, in’t Zandt and colleagues confirm well-established olfactory circuits, such as feedback projections form the PC and AON to the OB. However, their use of various retrograde tracing techniques failed to reveal a direct connection from the OT to the OB. The authors discuss their observations in the context of the experimental approaches used in prior studies. The results presented suggest that, unlike the direct influence in OB processing by the PC and AON, the influence of the OT on olfactory function is likely to occur at a higher-order processing level. The authors’ neuroanatomical analysis will help further the understanding of the role of the OT in odor perception. Instead of directly modulating the OB, the OT may act as a multimodal integrator, which serves to link contextual odor information with ongoing odor processing in other OCs. Future experiments should address this possibility by examining areas targeted by OT neurons using the combination of retrograde tracing techniques that in’t Zandt and colleagues used in this study.
This Reader's Pick was reviewed and edited by eNeuro Features Editor Rosalind S.E. Carney, D.Phil.
Read the full article:
Centrifugal Innervation of the Olfactory Bulb: A Reappraisal
Estelle E. in ’t Zandt, Hillary L. Cansler, Heather B. Denson and Daniel W. Wesson
FOLLOW US
POPULAR POSTS
TAGS
CATEGORIES


 RSS Feed
RSS Feed




