Remembering to Eat: Inhibiting Hippocampal Neural Activity during the Postprandial Period Increases Future Food Intake
Benjamin M. Seitz, M.A. is currently a PhD student at University of California, Los Angeles. Follow them on Twitter @benjamin_seitz
What makes an animal eat? The dominant model has been that of a homeostatic regulatory system that signals to an organism that it is nutrient deprived and should thus consume more food. There is no doubt such a system exists, but it is increasingly coming under scrutiny. For instance, if we simply eat because we have detected a lack of nutrients, why do people overeat during meals or eat when they are not nutrient deprived? Clearly, there are other motivating factors that stimulate people to initiate eating bouts.
A number of researchers now suggest that the memory of eating a recent meal influences the amount of food consumed at a later eating event. Suggestive of this, procedures that enhance memories of eating in human participants reduces later food consumption and, conversely, procedures that reduce memories of eating increases subsequent food consumption. In their eNeuro publication, Hannapel and colleagues provide further evidence for this latter pattern – inhibiting memory consolidation of a preceding meal increases subsequent food consumption.
Using optogenetics, a technique that allows for precision in temporarily inactivating brain regions, the authors were able to separately inactivate dorsal (dHC) and ventral (vHC) hippocampus (Figure 1) before, during, or after consumption of a meal (Figure 2). These brain regions were selected as they are involved in memory consolidation. The authors used a rigorous within-subjects design in which both the dHC and vHC were separately inactivated in each rat at each timepoint relative to the first meal. This generated seven experimental conditions: no inhibition, inhibition of dHC or vHC before, during, or after the first meal.
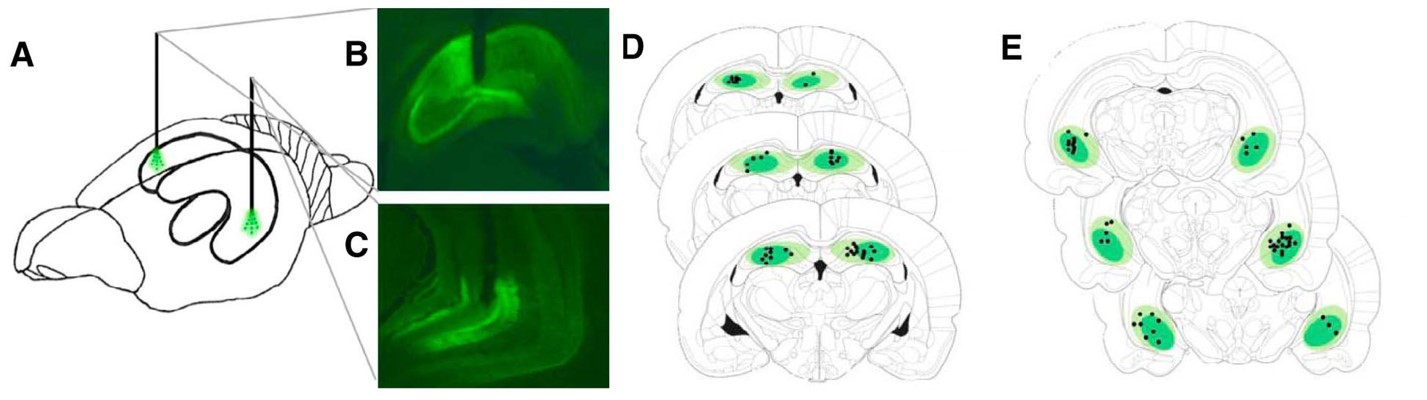
Figure 1. Viral expression and fiber location were used to confirm the location of optogenetic inhibition. (A) Depiction of optogenetic fiber placement in dHC and vHC of the same rat. (B,C) Representative image of robust viral expression and fiber placement location in dHC (B) and in (C) vHC. (D,E) Schematic depiction of coronal brain sections showing viral expression and fiber placement location placement in the dHC (D) and vHC (E). (Adapted from Figure 2 in Hannapel et al. 2019)
____________________________________________________________________________________________
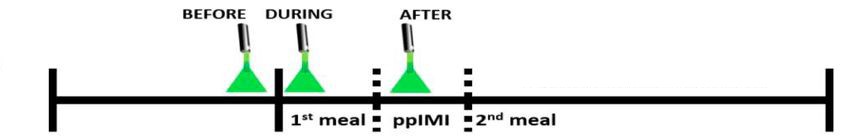
Figure 2. Timeline of optogenetic inhibition. Optogenetic inhibition of the dHC or vHC was performed before, during, or after consumption of the 1st meal. The temporal effect of neuronal inhibition was determined by assessing the onset and size of the 2nd meal with respect to the 1st meal. (Adapted from Figure 3 in Hannapel et al. 2019)
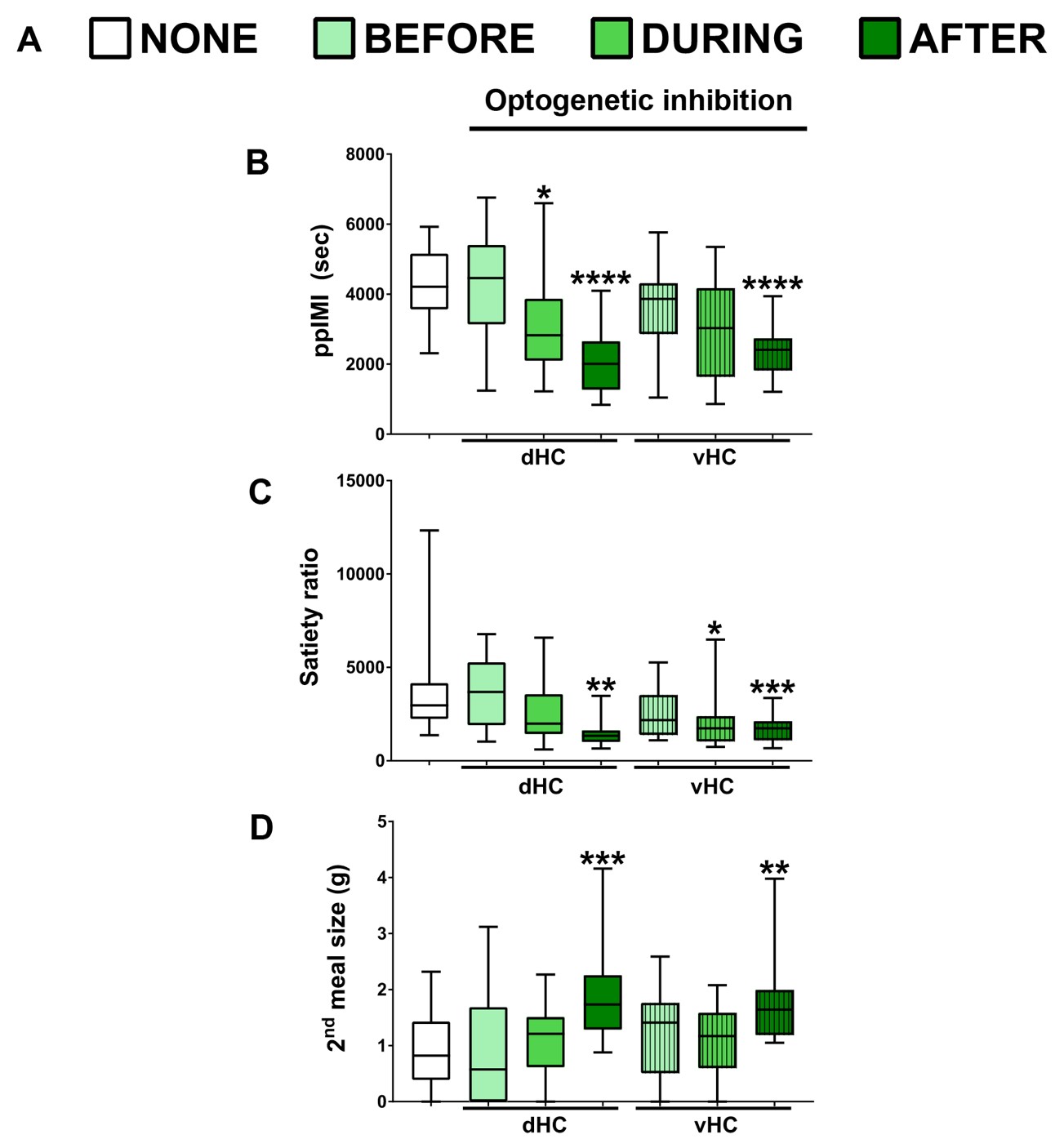
Hannapel and colleagues determined the effect of optogenetic inhibition on the latency to initiate a second meal and the amount of food consumed during the second meal. When the dHC or vHC was inactivated immediately after the first meal, rats initiated the second meal sooner than when inhibition was not given, which is reflected in the shorter postprandial intermeal interval (ppIMI) and lower satiety ratio (ppIMI/size of first meal). When the dHC or vHC were inactivated immediately after the first meal, rats also consumed more food during the second meal that occurred later when the neurons were no longer inhibited. This effect on the size of the next meal did not occur when the dHC or vHC was inactivated before or during the first meal or in rats given a control virus. Thus, inhibiting memory consolidation of an eating event accelerated the onset and the amount of food consumed during the next meal (Figure 3).
This effect was found when the first meal consisted of a sucrose solution, standard chow, or saccharin, a sweet substance that lacks calories. This latter finding is important because it suggests that the inhibition disrupted memory rather than the ability to sense calories. Therefore, understanding the factors that affect memory consolidation following a meal could potentially be used as a target for clinical intervention.
Figure 3.Timeline of optogenetic inhibition and effects on subsequent food consumption. (A) Experimental conditions. (B-D) Postmeal inhibition of dHC or vHC shortened the postprandial intermeal interval (ppIMI) (B), reduced the satiety ratio (ppIMI/size of first meal) (C) and increased the amount of food consumed during the 2nd meal (D). Asterisks indicate statistical significance compared to a day in which rats were attached to the laser but not given inhibition (i.e., NONE). (Adapted from Figure 4 in Hannapel et al. 2019)
Read the full article:
Postmeal Optogenetic Inhibition of Dorsal or Ventral Hippocampal Pyramidal Neurons Increases Future Intake
Reilly Hannapel, Janavi Ramesh, Amy Ross, Ryan T. LaLumiere, Aaron G. Roseberry and Marise B. Parent
FOLLOW US
POPULAR POSTS
TAGS
CATEGORIES


 RSS Feed
RSS Feed




