Effects of Status Epilepticus and Electroconvulsive Shock on Mouse Hippocampal Microglia
Atehsa Sahagun is currently an undergraduate student in biology & neuroscience at Santa Clara University, Santa Clara, CA.
An epileptic episode could strike anytime, leaving someone on the floor with involuntary, uncontrollable muscle movements and a potentially altered mental status. According to the World Health Organization (2019), epilepsy affects approximately 50 million people worldwide, and yet the mechanisms involved in the development of epilepsy (epileptogenesis) are, to date, not fully understood. Investigating further the role of microglia, the central nervous system’s resident macrophages, might result in more knowledge regarding the underlying mechanisms of epileptogenesis.
Status epilepticus (SE) seizures are known to activate hippocampal microglia following neuronal hyperactivity and neuronal damage. SE occurs when a single seizure lasts more than 5 minutes or more than one seizure occurs in 5 minutes without recovery between seizures (Epilepsy Foundation, 2014). Because SE results in both neuronal hyperactivity and neuronal damage, the exact trigger for microglial activation, or whether activation is a consequence of SE-induced damage, is unclear. In their eNeuro publication, Sepulveda-Rodriguez and colleagues used electroconvulsive shock (ECS), a model of acute seizure, in mice to examine hippocampal CA1 microglial activation and physiology in response to neuronal hyperactivity, independent of epileptogenesis or neuronal damage.
Electroconvulsive shock therapy is currently used as a last resort for individuals with severe depression or mood disorders; however, the molecular mechanisms that underlie ECS therapy remain a mystery. Sepulveda-Rodriguez and colleagues examined microglial morphology and physiology 24 hours after SE or ECS induction in adult mice with green fluorescent protein-labeled microglia. SE was induced by pilocarpine injection, and ECS was induced by transcorneal stimulation; control mice received sham shock or injection of saline/vehicle.
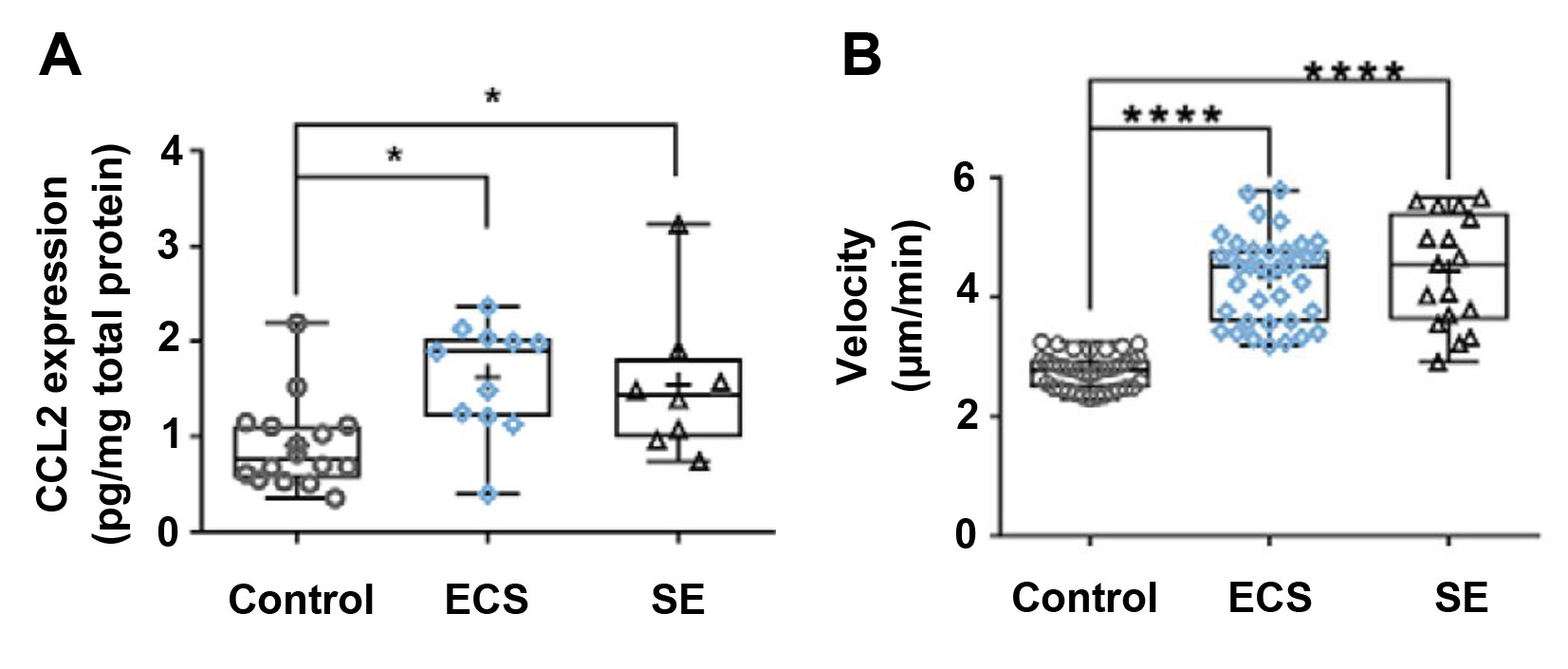
Use of a marker of neuronal damage confirmed that hippocampal neuronal damage followed induction of SE, but not ECS. The results of microglial morphological analyses were indicative of microglial activation in the SE group but in not the ECS or control groups. CCL2 is a chemokine produced in sites of inflammation; increased CCL2 expression is associated with SE and neuronal damage. Therefore, the authors expected that CCL2 expression would be lower in ECS mice, compared to SE mice. However, they found that CCL2 expression in the CA1 region of ECS mice was similar to the expression levels observed in SE mice (Figure 1A). As CCL2 was already known to upregulate purinergic signaling in microglia, the authors next examined microglial responsive motility, which is a measure of purinergic signaling. Following the application of 3 mM ATP to acute hippocampal slices with a patch pipette, the velocity of microglial processes in the ECS and SE groups were found to be significantly higher than that of the control (Figure 1B). These findings indicate that ECS increases purinergic responses within microglia.
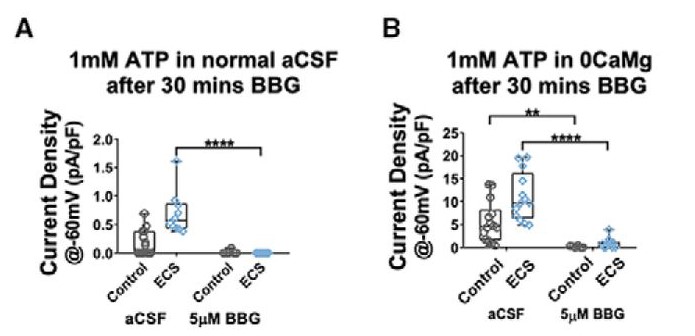
Sepulveda-Rodriguez and colleagues performed further electrophysiology experiments and found that ECS did not alter intrinsic electrophysiological characteristics of CA1 microglia. The ECS condition showed increased cationic current in the presence of ATP, compared to control currents, indicating that there was increased ionic conductance after ECS treatment. Current density was increased in the ECS group compared to the control. Current density is a readout of receptor number per cell, and based on channel kinetics, the authors hypothesized that the receptors were purinergic. To confirm that the change in cation current found after ECS treatment in microglia was due specifically to purinergic receptor conductance, Brilliant Blue G (BBG), a purinergic inhibitor, was added to hippocampal slices. Blocking P2X7 purinergic receptors resulted in a significant decrease in the current density in both artificial cerebrospinal fluid (aCSF) and cation-free ECS conditions compared to the control (Figure 2). These findings indicate that P2X7 receptors are responsible for the change in microglial ionic currents after ECS. P2X7-containing receptors are known to have permeability characteristics that contribute to their role in epilepsy and neuroinflammation. The authors examined expression levels of genes that encode purinergic receptors; these results show that P2X7-mediated changes in current density following ECS arise post-transcriptionally.
In their eNeuro publication, Sepulveda-Rodriguez and colleagues describe similarities and differences between the microglial physiological changes following ECS-induced, non-epileptogenic acute seizure and SE-induced epileptogenic seizure that is accompanied by neuronal damage. Specifically, the authors identify a mechanism for the change in microglial activation via purinergic receptor current density changes after ECS- and SE-induced seizure. These observations may provide valuable insight into the mechanism of electroconvulsive shock therapy. Gaining a better understanding of microglial activation following an ECS- or SE-induced seizure could potentially have profound implications for individuals with epilepsy, severe depression, or mood disorders.
Read the full article:
Electroconvulsive Shock Enhances Responsive Motility and Purinergic Currents in Microglia in the Mouse Hippocampus
Alberto Sepulveda-Rodriguez, Pinggan Li, Tahiyana Khan, James D. Ma, Colby A. Carlone, P. Lorenzo Bozzelli, Katherine E. Conant, Patrick A. Forcelli and Stefano Vicini
This Reader's Pick was reviewed and edited by eNeuro Features Editor Rosalind S.E. Carney, D.Phil.
References:
- Epilepsy Foundation (2014) Status Epilepticus. https://www.epilepsy.com/learn/challenges-epilepsy/seizure-emergencies/status-epilepticus March 19th, 2014; retrieved August 21st, 2019.
- World Health Organization (2019) Fact sheet: epilepsy. https://www.who.int/news-room/fact-sheets/detail/epilepsy Updated June 20th, 2019; retrieved August 18th, 2019.
FOLLOW US
POPULAR POSTS
TAGS
CATEGORIES


 RSS Feed
RSS Feed




