Scd-2 Expression Mediates Worm Learning and Sensory Integration
Dr. Snider is currently a postdoctoral researcher at Yale University, New Haven, CT. Follow them on Twitter @KH_Snider
Learning—simply put, the ability of an organism to change its behavior due to experience—is a conserved phenomenon from tardigrades to humans. Much of our knowledge of the molecular and cellular mechanisms of learning was first discovered in invertebrate model organisms. Caenorhabditis elegans, a tiny nematode worm, is an invertebrate model with many advantages for neuroscience experiments: its neuronal activity can be imaged without surgical intervention, and its genetics makes it very tractable for neuron-specific transgenic manipulations, such as optogenetics and gene rescue experiments. However, comparatively little is known about the molecular basis of learning in C. elegans. The two most widely-known learning-associated mutations in the worm—lrn-1 and lrn-2—unidentified within the genome for over twenty years. In their eNeuro publication, Wolfe and colleagues show that the lrn-2 mutation is allelic to the scd-2 gene, which codes for a receptor tyrosine kinase, and examined scd-2 function in associative learning and sensory integration.
The authors used a combination of sequencing, complementation, and rescue experiments to demonstrate that the mutation lrn-2 is a recessive loss-of-function mutation in the scd-2 gene. SCD-2 is homologous to the mammalian protein anaplastic lymphoma kinase, which is involved in learning and memory in mammals. Several behavioral assays have been used to test deficits in worms with the lrn-2 mutation. Associative learning simply pairs two stimuli (one aversive or punishing, and one attractive) for a period of time. Wild-type worms from a reference strain show reduced attraction to an odor after that odor is paired with an aversive stimulus; scd-2 mutant worms fail to show this learning. While associative learning assays change behavior after a period of time, sensory integration assays alter behavior immediately. A sensory integration assay combines a repellent stimulus (such as copper acetate) with an attractive stimulus (such as diacetyl). Just as a human might only go out in the rain if they know there is free pizza across the street, wild-type worms will cross an aversive copper barrier if there is diacetyl on the other side. While lrn-2 mutant worms have typical unisensory responses (they avoid copper acetate and are attracted to diacetyl), they fail to alter their response when both are presented together.
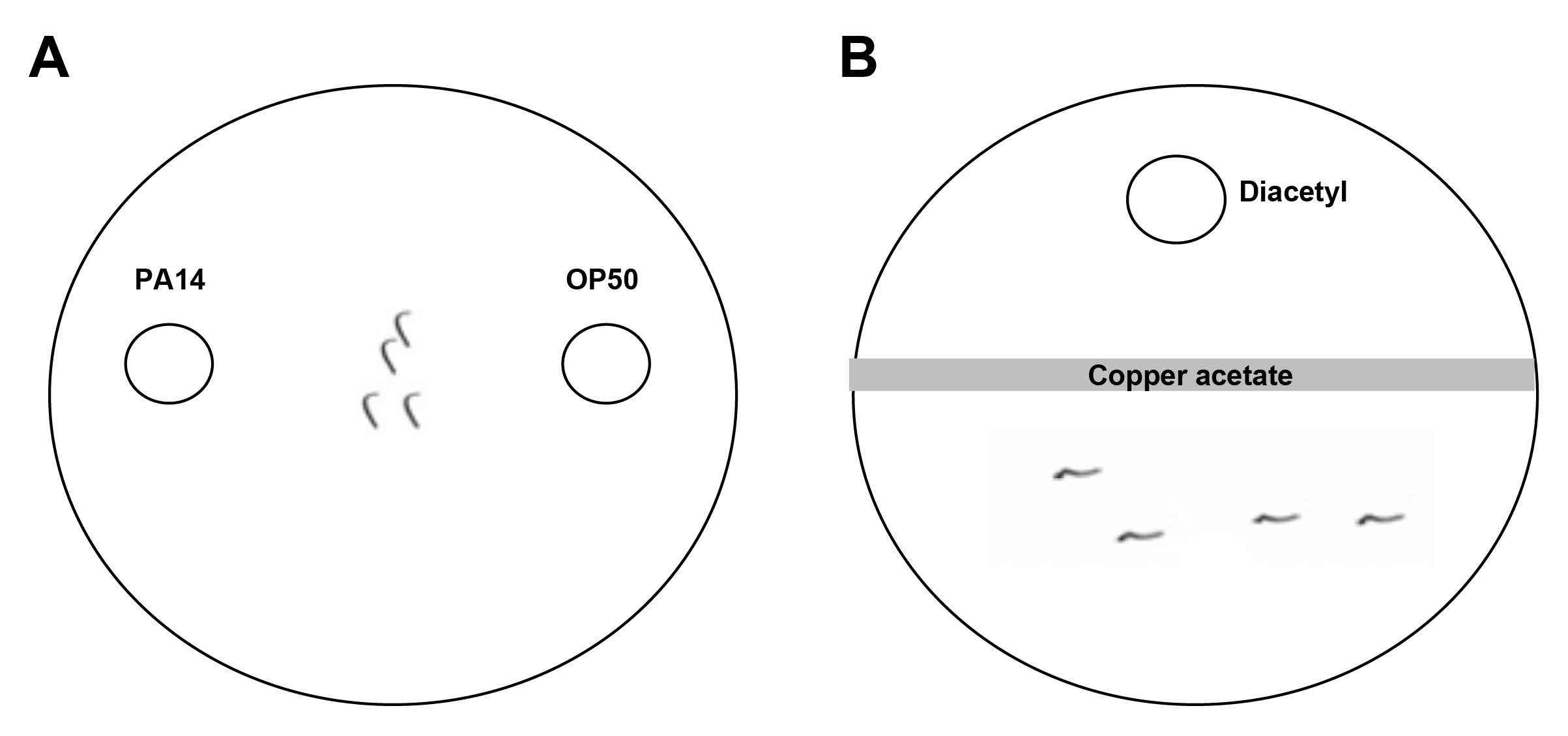
Wolfe and colleagues were interested in localizing the role of scd-2 expression in these behavioral processes to specific neurons. One of the traits that make worms so tractable to neuroscience research is that we can control the expression of genes in an extremely small number of neurons: in these cases, just two neurons at a time (the AIA neuron pair, or the NSM neuron pair). The authors tested localization of scd-2 expression in a form of associative learning in which worms learn to avoid a pathogenic bacterium, Pseudomonas aeruginosa (PA14). Naïve worms are attracted to PA14 and eat it readily; however, PA14 then makes the worms ill. Thus, they learn to pair the PA14 odor with pathogenicity, an indicator of associative learning. Worms that have previously eaten PA14 will subsequently avoid PA14 and move toward a non-pathogenic bacterium, OP50 Escherichia coli when the worms are replated on a Petri dish containing a spot of either bacterium on opposite sides (Figure 1A). For the sensory integration assay, worms were placed on an agar plate on one side of a copper barrier; worms are known to avoid copper. The other side of the plate was spotted with diacetyl, an odor that worms are attracted to. Worms that cross the barrier to the location of diacetyl exhibit normal sensory integration (Figure 1B). The authors focused on the AIA and NSM neuron pairs, which are involved in sensory integration and PA14 avoidance learning, respectively. The authors restored wild-type scd-2 expression in AIA interneurons or NSM neurons in worms that had scd-2 mutant backgrounds.
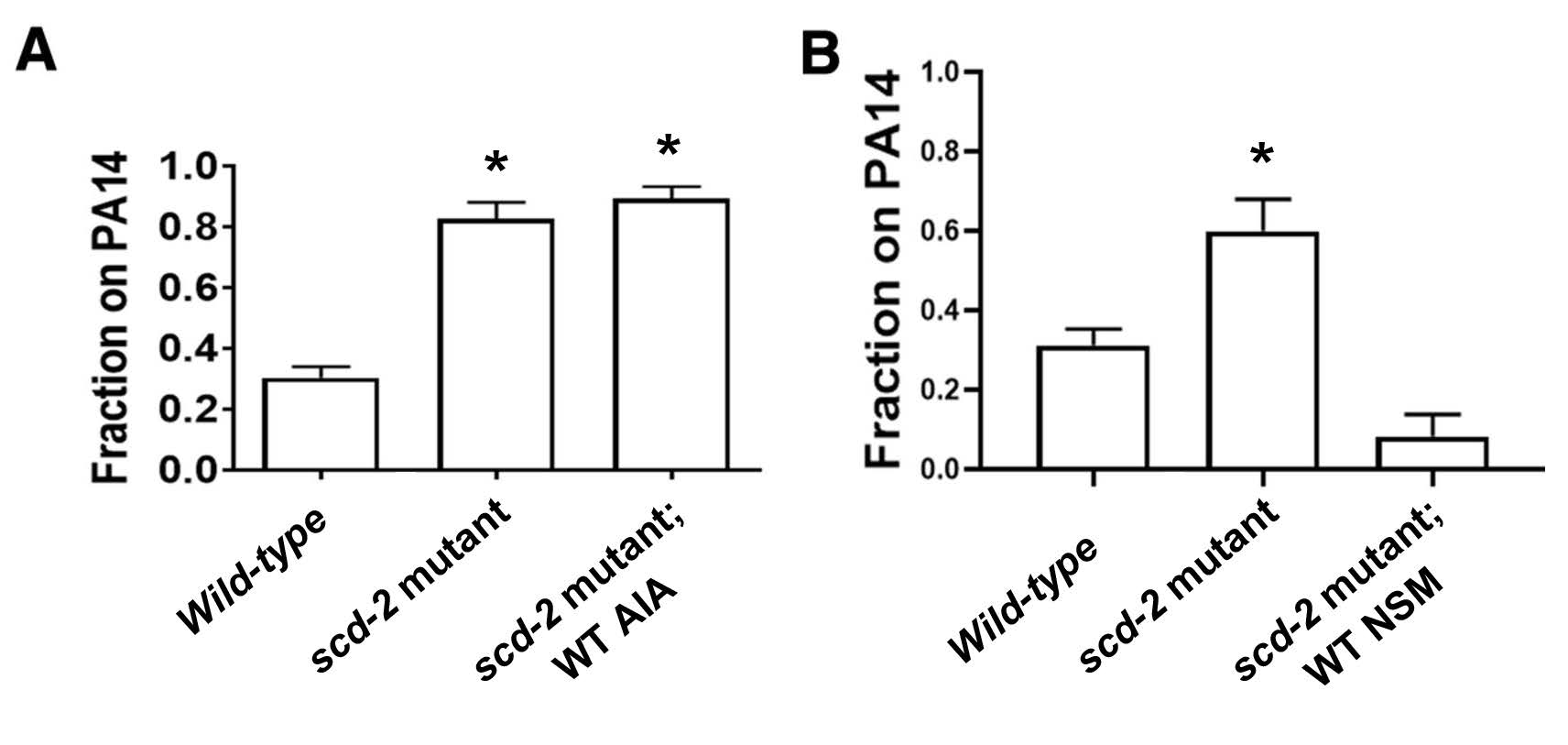
For the associative learning assay, the fraction of worms on the PA14 spot was calculated; a smaller fraction indicates that the experimental group of worms learned the association between odor and PA14 pathogenicity. As expected, worms from a wild-type reference strain avoided the PA14 spot, but scd-2 mutant worms are unable to learn to avoid PA14. When scd-2 expression was restored in AIA neurons, worms with an scd-2 mutant background still failed to learn to avoid PA14 (Figure 2A). In contrast, restoration of scd-2 expression in NSM neurons was sufficient for worms with an scd-2 mutant background to learn to avoid PA14 (Figure 2B). These observations indicate that the role of scd-2 in associative learning is specific to NSM neurons.
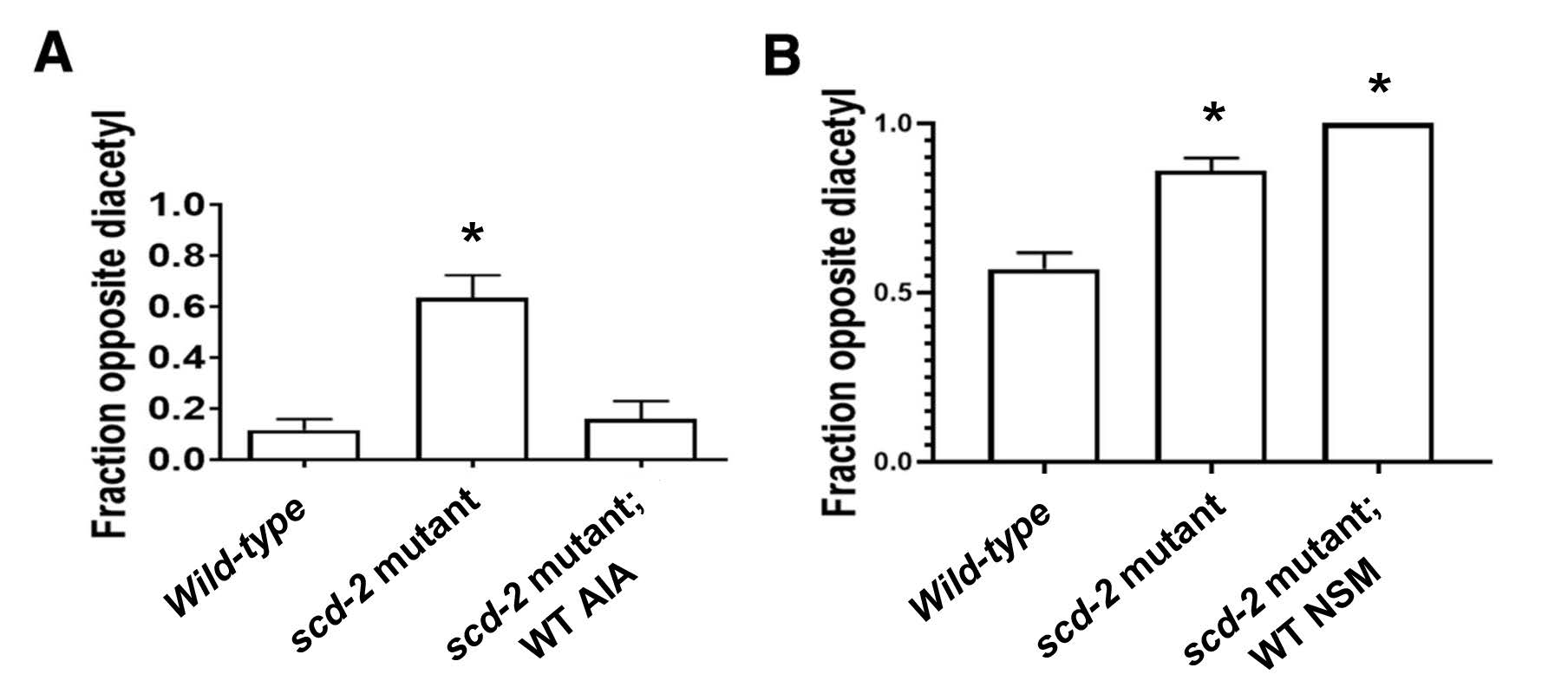
For the sensory integration assay, the fraction of worms opposite diacetyl was calculated; a smaller fraction indicates that the experimental group of worms had integrated the sensory cues and cross over the copper barrier to reach the diacetyl spot. Wild-type worms cross the copper barrier and congregate on the diacetyl side. scd-2 mutant worms fail to cross the copper barrier, even though they are normally attracted to diacetyl. However, the authors found that expression of wild-type scd-2 in the AIA neuron pair was sufficient to restore normal sensory integration in worms with an scd-2 mutant background (Figure 2A). Restoration of scd-2 expression in the NSM neuron pair in worms with an scd-2 mutant background did not rescue the inability to integrate the sensory cues; a large proportion of these worms did not cross the copper barrier (Figure 2B). These observations indicate that the role of scd-2 in sensory integration is specific to AIA neurons.
Identifying scd-2 as a gene necessary for worm learning is useful; first, because it implicates a variety of other upstream and downstream proteins that may also play a role in learning. These associated proteins are now likely candidates for further studies. Second, the fact that the same protein is involved in both learning and sensory integration, yet through different neurons (and, as further expanded in the paper, different molecular pathways), is very interesting. These findings suggest that integration of an animal’s past and current experience may have originally worked via common pathways that later diverged and became specialized to allow for finer tuning of behavior. These results are an important contribution to the knowledge of molecular mechanisms of worm learning and sensory integration that may be applicable to learning in many organisms.
Read the full article:
A Receptor Tyrosine Kinase Plays Separate Roles in Sensory Integration and Associative Learning in C. elegans
Glenn S. Wolfe, Vivian W. Tong, Emily Povse, Daniel M. Merritt, Gregory W. Stegeman, Stephane Flibotte and Derek van der Kooy
This Reader's Pick was reviewed and edited by eNeuro Features Editor Rosalind S.E. Carney, D.Phil.
References:
- Ishihara T, Iino Y, Mohri A, Mori I, Gengyo-Ando K, Mitani S, Katsura I (2002) HEN-1, a secretory protein with an LDL receptor motif, regulates sensory integration and learning in Caenorhabditis elegans. Cell 109:639–649. doi:10.1016/S0092-8674(02)00748-1
- Shinkai Y, Yamamoto Y, Fujiwara M, Tabata T, Murayama T, Hirotsu T, Ikeda DD, Tsunozaki M, Iino Y, Bargmann CI, Katsura I, Ishihara T (2011) Behavioral choice between conflicting alternatives is regulated by a receptor guanylyl cyclase, GCY-28, and a receptor tyrosine kinase, SCD-2, in AIA interneurons of Caenorhabditis elegans. J Neurosci 31:3007–3015. doi:10.1523/JNEUROSCI.4691-10.2011
- Zhang Y, Lu H, Bargmann CI (2005) Pathogenic bacteria induce aversive olfactory learning in Caenorhabditis elegans. Nature 438:179–184. doi:10.1038/nature04216
FOLLOW US
POPULAR POSTS
TAGS
CATEGORIES


 RSS Feed
RSS Feed




