TrkB Function in Cortistatin-expressing Interneurons Regulates Cortical Gene Expression Patterns
Catherine Smoot is currently a PhD Candidate at West Virginia University, Morgantown, WV. Follow them on Twitter @catherine_smoot
Inhibitory interneurons of the cerebral cortex are critical for cognitive and executive function. At the circuit level, inhibitory (GABAergic) interneurons regulate the activity of excitatory projection neurons to achieve an appropriate excitatory/inhibitory (E/I) balance. Interneuron dysfunction is implicated in several neurological disorders, including schizophrenia, autism spectrum disorder, and epilepsy. Different interneuron subtypes are defined by their neurochemical expression, for example, parvalbumin, somatostatin (SST), and cortistatin (Cort). Cort and SST interneurons share similarities in their structural and functional properties, namely, the ability to bind to SST receptors and to depress neural activity. However, Cort is produced from a different gene locus than SST and carries out distinct roles such as inducing slow-wave sleep activity and regulating distinct aspects of synaptic transmission.
Brain-derived neurotrophic factor (BDNF) is expressed in excitatory cortical neurons and is secreted in response to neuronal activity. BDNF is neither expressed in nor secreted by inhibitory interneurons. Expression of tropomyosin receptor kinase B (TrkB), the cognate receptor for BDNF, is, however, found in most inhibitory interneurons. Thus, via BDNF, excitatory neurons signal in a paracrine manner to interneurons expressing TrkB. Loss of BDNF-TrkB signaling causes a downregulation in the expression of many genes associated with cortical interneuron function, including the gene that encodes Cort (Martinowich et al., 2011; Guilloux et al., 2012). Research by the lab of Professor Keri Martinowich (Lieber Institute for Brain Development, Johns Hopkins School of Medicine, Baltimore, MD) has previously shown that conditional deletion of the TrkB receptor in Cort interneurons results in hyperactivity and fatal seizures in mice (Hill et al., 2019). In their eNeuro publication, Maynard and colleagues examined the molecular mechanisms of dysregulated Cort interneuron function that result from conditional deletion of TrkB in Cort cells.
The authors used a previously generated Cre-lox mouse model to conditionally delete TrkB in Cort cells (Hill et al., 2019). Bulk RNAseq of RNA isolated from whole cortex homogenate samples of control and conditional knockout mice was performed to identify and quantify changes in gene expression levels in the surrounding cellular milieu. To investigate gene expression changes within Cort interneurons, the authors genetically introduced a RiboTag allele to isolate ribosome-bound RNA selectively in Cort interneurons. Using this translating ribosome affinity purification (TRAP) approach, they compared RNA expression patterns in Cort interneurons with (control) and without (conditional deletion) intact TrkB receptor expression.
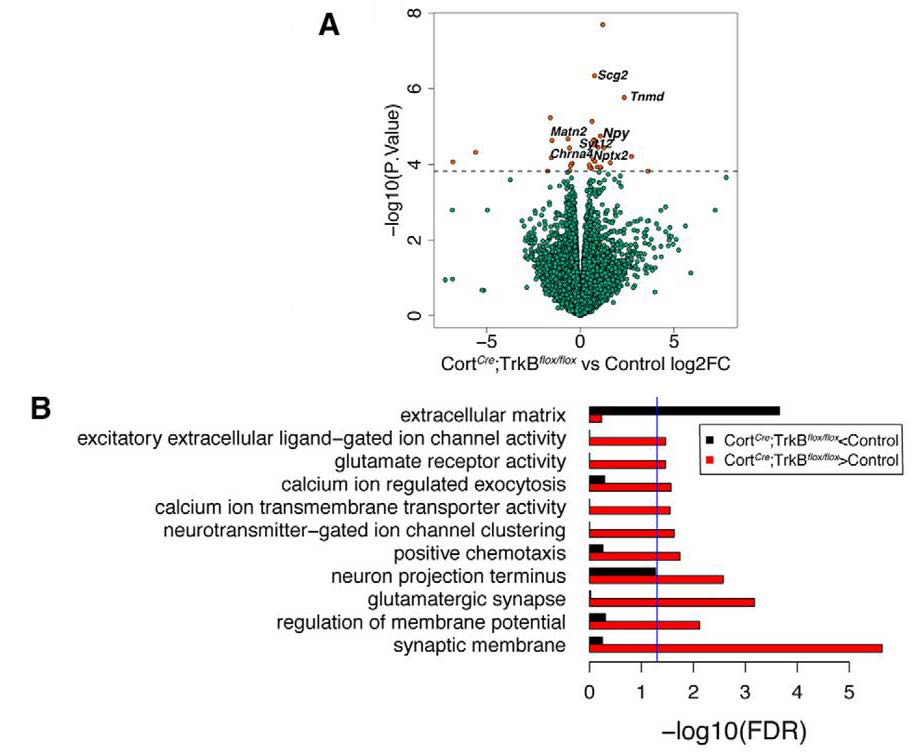
Despite experimentally deleting TrkB receptor expression selectively in Cort interneurons, the authors observed surprising indirect changes in the expression of genes related to excitatory neuron function in the bulk RNA-seq analysis (Figure 1). These findings suggest that TrkB signaling in Cort interneurons non-autonomously influences gene expression in excitatory neurons. This observation is exciting because it highlights the intimate molecular interconnectedness of excitatory and inhibitory interneurons. Complementing the lab’s previous work that identified a spontaneous seizure phenotype in TrkB conditional knockout mice (Hill et al., 2019), loss of TrkB expression from Cort interneurons also resulted in increased transcription of activity-dependent genes, including Bdnf itself. These changes could be a direct consequence of early seizures in mice lacking TrkB expression. Another possibility is a compensatory mechanism in which excitatory neurons attempt to counterbalance the absence of TrkB receptor expression in Cort interneurons, and in response, upregulate BDNF expression. The activity-dependent genes that underwent transcriptional changes are known to contribute to a positive-feedback loop that regulates neuronal hyperactivity and affects seizure severity and duration (Hu and Russek, 2008).
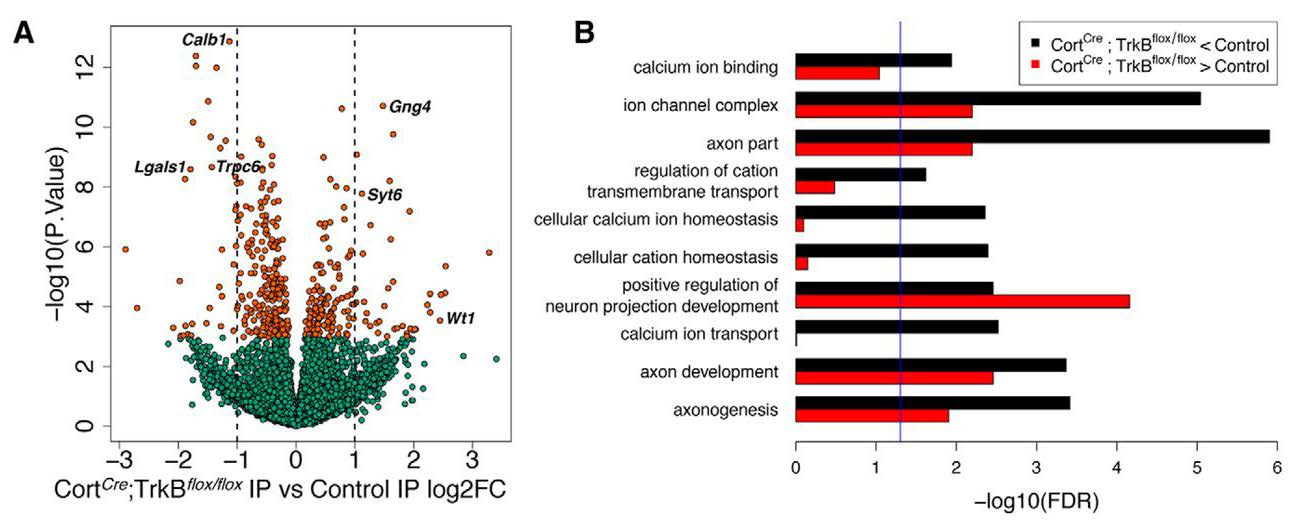
Using the Ribotag approach to directly profile Cort interneurons, the authors also found that loss of TrkB expression within these interneurons resulted in significant decreases in the expression of genes related to calcium signaling (Figure 2). As calcium signaling is important for neurotransmission and multiple signaling pathways, Cort interneuron-mediated alterations in calcium levels may contribute to an E/I imbalance. Maynard and colleagues also found a significant bidirectional dysregulation of genes associated with axon development when TrkB was conditionally deleted in Cort interneurons (Figure 2). Axon development is crucial for creating the appropriate connections with target cells in the cortical circuit. If this function is compromised, Cort neurons may never reach their target cells and/or could form synapses with incorrect targets. This scenario could result in aberrant inhibition of excitatory neurons by Cort interneurons, leading to the cortical hyperexcitability phenotype that the authors previously described (Hill et al., 2019).
This publication is an advance in the field because it reveals several findings that highlight the diversity of Cort interneuron function in the mouse cortex. For example, this research provides evidence of the molecular communication that occurs between neighboring cell types that is needed to build a mature cortical circuit that functions with a correct E/I balance. Maynard and colleagues’ results indicate that the TrkB-BDNF-dependent signaling is not only important for inhibitory interneuron development, but also for regulating the function of excitatory neurons. Future work may identify the cellular and molecular mechanisms by which BDNF-TrkB signaling in Cort interneurons can indirectly regulate excitatory neuron activity.
The authors also found changes in gene expression related to calcium signaling and axon development that resulted from the absence of TrkB expression in Cort interneurons. These alterations in gene expression suggest that the absence of TrkB in Cort interneurons results in modifications of molecular, physiological, and connectivity patterns. However, their lab previously reported no evidence for cell-autonomous changes in the number, distribution, molecular expression patterns, or intrinsic properties of Cort interneurons after conditional removal of TrkB (Hill et al., 2019). Even if the number of Cort interneurons is unchanged in the absence of TrkB expression, perhaps the number of Cort synapses is compromised. This question can be assessed by comparing the density of Cort synapses on target cells after conditional deletion of TrkB. Alternatively, the laminar distribution of Cort synapses may be aberrant, which could be examined by visualization of Cort interneuron morphology and dendritic arborization complexity.
Interestingly, the authors found non-autonomous gene expression changes across the whole cortex following conditional deletion of TrkB in Cort interneurons. Specifically, Maynard and colleagues found differential regulation of glutamatergic synapses and synaptic membranes, indicating an indirect effect of the absence of TrkB in Cort interneurons on the excitatory function of local neurons. This finding pairs nicely with the reduced excitatory drive onto Cort interneurons following conditional deletion of TrkB, as quantified by decreased frequency of spontaneous excitatory postsynaptic potentials in layer II/III Cort interneurons (Hill et al., 2019). Taken together, there is mounting evidence of the critical intercellular communication necessary for generating functional cortical circuitry. Understanding the changes in the expression of cortical genes affected by Cort interneuron dysfunction may provide insights into potential novel therapeutic targets for neurological disorders, particularly those characterized by cortical hyperexcitability and seizure activity.
This Reader's Pick was reviewed and edited by eNeuro Features Editor Rosalind S.E. Carney, D.Phil.
References:
- Guilloux J-P, Douillard-Guilloux G, Kota R, Wang X, Gardier A, Martinowich K, et al. Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with Major Depression.Mol Psychiatry. 2012 Nov;17(11):1130–42.
- Hill JL, Jimenez DV, Mai Y, Ren M, Hallock HL, Maynard KR, et al. Cortistatin-expressing interneurons require TrkB signaling to suppress neural hyper-excitability.Brain Struct Funct.2019 Jan;224(1):471–83.
- Hu Y, Russek SJ. BDNF and the diseased nervous system: a delicate balance between adaptive and pathological processes of gene regulation. J Neurochem.2008 Apr;105(1):1–17.
- Martinowich K, Schloesser RJ, Jimenez DV, Weinberger DR, Lu B. Activity-dependent brain-derived neurotrophic factor expression regulates cortistatin-interneurons and sleep behavior. Mol Brain.2011 Mar 9;4:11.
Read the full article:
TrkB Signaling Influences Gene Expression in Cortistatin-Expressing Interneurons
Kristen R. Maynard, Alisha Kardian, Julia L. Hill, Yishan Mai, Brianna Barry, Henry L. Hallock, Andrew E. Jaffe, and Keri Martinowich
FOLLOW US
POPULAR POSTS
TAGS
CATEGORIES


 RSS Feed
RSS Feed




