Early Biomarker for Motor Skill Consolidation Defects in Huntington’s Disease Mouse Model
Julia Badyna is currently a PhD student in biological sciences at Carnegie Mellon University in Pittsburgh, PA.
Huntington’s Disease (HD) is a neurodegenerative disorder that leads to pronounced motor defects and alterations in behavior and cognitive function. HD is caused by a CAG repetition in the gene that encodes the variant huntingtin (HTT) protein. Variant HTT is associated with aberrant excitatory neurotransmission, neurotoxicity, and, ultimately, the death of medium spiny neurons (MSNs) of the striatum. The neurodegeneration and death of MSNs results in the overt motor defects on which a diagnosis of HD (fully progressed) is typically based. However, individuals with HD exhibit cognitive defects and initial motor impairments at an early (premanifest) stage relative to conventional diagnosis.
Research in rodent models of HD has shown that physiological changes at MSN striatal synapses are present in fully progressed HD1,2. However, changes in NMDA receptor-mediated synaptic transmission are already evident in the early stages of HD5. NMDA receptor subunit composition changes affect excitatory transmission in several neurological diseases6. HD is associated with changes in expression of the GluN2B and GluN3A subunits of NMDA receptors3,4. However, it was unknown whether the alterations in NMDA subunit expression were related to early motor deficits. In their publication, Glangetas and colleagues examined whether aberrant synaptic plasticity may be responsible for, and serve as an early biomarker of, motor skill consolidation deficits in a mouse model of premanifest HD.
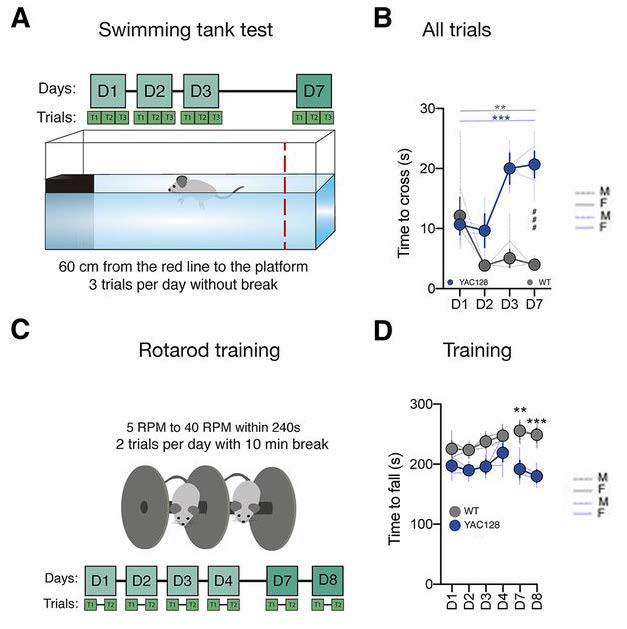
YAC128 mice are an established mouse model for HD; gross locomotor impairment is typically evident at ten months. Mild cognitive defects prior to the onset of gross locomotor impairments have been previously reported in young YAC128 mice7. Glangetas and colleagues, therefore, used young (11–14 weeks) YAC128 mice as a model of premanifest HD. Several locomotor tests were used to determine whether premanifest YAC128 mice exhibited motor defects compared to wild-type (WT) age-matched mice. In the swimming tank test, mice were trained to swim 60 cm to reach a platform in a series of trials over three days (Figure 1A). Whereas premanifest YAC128 mice learned the swimming task as well as WT mice on Day 1, they did not show a comparable improvement in performance on days 2 and 3 demonstrated by WT mice (Figure 1B). Testing on Day 7 revealed that premanifest YAC128 mice displayed a floating behavior not observed in WT mice, and premanifest YAC128 mice failed to reach the platform in a number of trials (Figure 1B). The observations demonstrate that premanifest YAC128 mice show defects in motor skill consolidation at a much earlier age than the gross locomotor defects described in older YAC128 mice.
The authors also examined locomotor activity using the rotarod test. Mice were placed on a circular metal rod that rotated at an increasing number of revolutions per minute (Figure 1C). Compared to a short latency to fall off the rod, a longer latency demonstrates better motor skills. The mice were trained on the rotarod twice daily on days 1–4. No initial difference in learning ability was found between WT and premanifest YAC128 mice (Figure 1D). Testing on Day 7, however, showed that the latency to fall off the rod was shorter in premanifest YAC128 mice than WT mice (Figure 1D). These results further show that premanifest YAC128 mice demonstrate defects in motor skill consolidation, whereas initial learning of motor skills is not impaired in these mice.
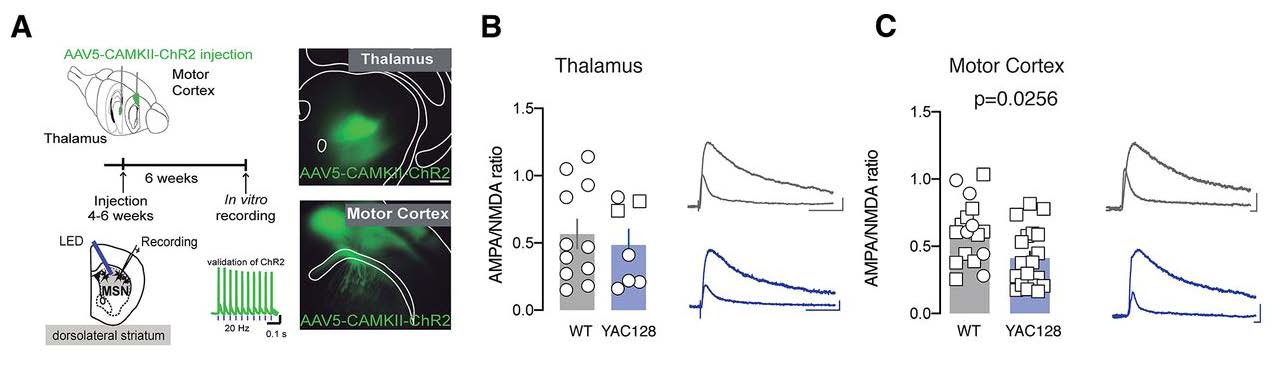
Next, the authors examined whether the premanifest YAC128 mice exhibit defects in excitatory synaptic transmission. The authors focused on the dorsolateral striatum (DLS), which is involved in the consolidation of motor skills, as opposed to the dorsomedial striatum, which contributes to the learning of motor skills. The authors visualized axonal projections using injection, in either the motor cortex or thalamus, of a viral construct that expresses a green fluorescence reporter protein (Figure 2A). This experimental approach enabled the authors to show, in slice recordings, a decreased AMPA/NMDA ratio specifically at motor cortex to DLS synapses in premanifest YAC128 mice. Determining the AMPA/NMDA ratio showed that aberrant NMDA transmission occurred in premanifest YAC128 mice. Moreover, this change was related to an alteration in NMDA receptor subunit composition; there was a significant increase in GluN2B subunits in NMDA receptors at motor cortex to DLS synapses in premanifest YAC128 mice. The presence of enriched GluN2B subunits was confirmed using Ifenprodil, an NMDA receptor inhibitor that acts specifically at GluN2B subunits. Ifenprodil had an increased effect on NMDA receptors in slices isolated from premanifest YAC128 mice compared to slices isolated from WT mice. By performing optogenetic experiments to evoke long-term depression, the authors concluded that deficits in premanifest YAC128 mice might include aberrant NMDA receptor transmission at motor cortex to DLS synapses leading to altered plasticity at these synapses (Figure 2C).
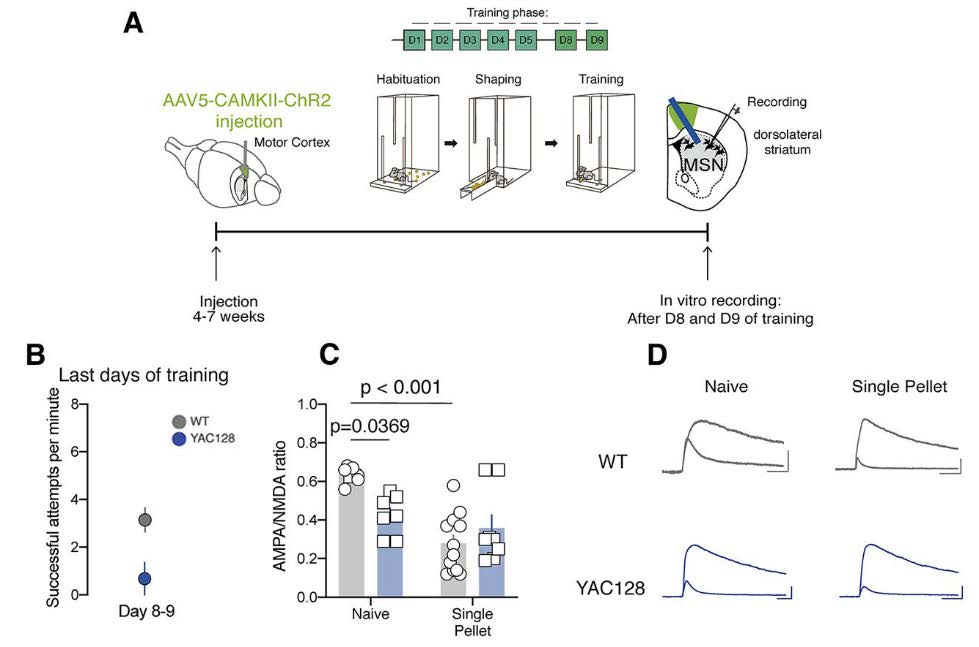
The authors next determined whether the physiological changes at NMDA receptors could be linked to the behavioral deficits in motor skill consolidation that were observed. The authors used the single pellet reaching task which requires fine motor coordination skills. As before, injection of a viral construct was used to visualize axonal projections from the motor cortex to the DLS (Figure 3A). On the last days of training, premanifest YAC128 mice were less successful at performing the single pellet reaching task than WT mice (Figure 3C). In WT mice, training on the single pellet reaching task decreased the AMPA/NMDA ratio at motor cortex to DLS synapses; surprisingly, this plasticity was not observed in the premanifest YAC128 mice (Figure 3C). The authors concluded that the consolidation-induced decrease in AMPA/NMDA ratio is occluded in these YAC128 mice. The AMPA/NMDA ratio at the motor cortex to DLS synapses in premanifest YAC128 mice is too low to support plasticity changes that normally occur during motor skill consolidation. Therefore, the authors concluded that motor cortex to DLS NMDA-mediated synaptic plasticity is necessary for the consolidation of newly acquired motor skills. Both the motor impairments and physiological alteration observed in premanifest YAC128 mice, therefore, serve as-early stage biomarkers of HD.
This publication provides important insights for both the HD and the motor learning research communities. The authors found locomotor behavioral and physiological changes that could serve as early biomarkers of HD in mice. The results serve as an exciting launch point for future work in early HD therapeutic intervention and also highlight the importance of motor cortex to DLS synapses for motor skill consolidation.
This Reader's Pick was reviewed and edited by eNeuro Features Editor Rosalind S.E. Carney, D.Phil.
References:
- Creus-Muncunill J, Ehrlich ME. Cell-Autonomous and Non-cell-Autonomous Pathogenic Mechanisms in Huntington's Disease: Insights from In Vitro and In Vivo Models. Neurotherapeutics.Neurotherapeutics.2019;16(4):957‐978. doi:10.1007/s13311-019-00782-9
- Kovalenko M, Milnerwood A, Giordano J, et al. HttQ111/+ Huntington's Disease Knock-in Mice Exhibit Brain Region-Specific Morphological Changes and Synaptic Dysfunction.J Huntingtons Dis. 2018;7(1):17‐33. doi:10.3233/JHD-170282
- Boussicault L, Kacher R, Lamazière A, et al. CYP46A1 protects against NMDA-mediated excitotoxicity in Huntington's disease: Analysis of lipid raft content.Biochimie.2018;153:70‐79. doi:10.1016/j.biochi.2018.07.019
- Marco S, Giralt A, Petrovic MM, et al. Suppressing aberrant GluN3A expression rescues synaptic and behavioral impairments in Huntington's disease models.Nat Med.2013;19(8):1030‐1038. doi:10.1038/nm.3246
- Milnerwood AJ, Gladding CM, Pouladi MA, et al. Early increase in extrasynaptic NMDA receptor signaling and expression contributes to phenotype onset in Huntington's disease mice.Neuron.2010;65(2):178‐190. doi:10.1016/j.neuron.2010.01.008
- Paoletti, P., Bellone, C. & Zhou, Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease.Nat Rev Neurosci.2013 (14):383–400. doi:10.1038/nrn3504
- Van Raamsdonk JM, Pearson J, Slow EJ, Hossain SM, Leavitt BR, Hayden MR. Cognitive dysfunction precedes neuropathology and motor abnormalities in the YAC128 mouse model of Huntington's disease.J Neurosci.2005;25(16):4169‐4180. doi:10.1523/JNEUROSCI.0590-05.2005
Read the full article:
Deficit in Motor Skill Consolidation-Dependent Synaptic Plasticity at Motor Cortex to Dorsolateral Striatum Synapses in a Mouse Model of Huntington’s Disease
Christelle Glangetas, Pedro Espinosa, and Camilla Bellone
FOLLOW US
POPULAR POSTS
TAGS
CATEGORIES


 RSS Feed
RSS Feed




